Molar Mass of Cl
Has one atom of sodium Na and one atom of chlorine Cl. The total should be 100 percent.

Molar Mass Molecular Weight Of Cl2 Chlorine Gas Youtube
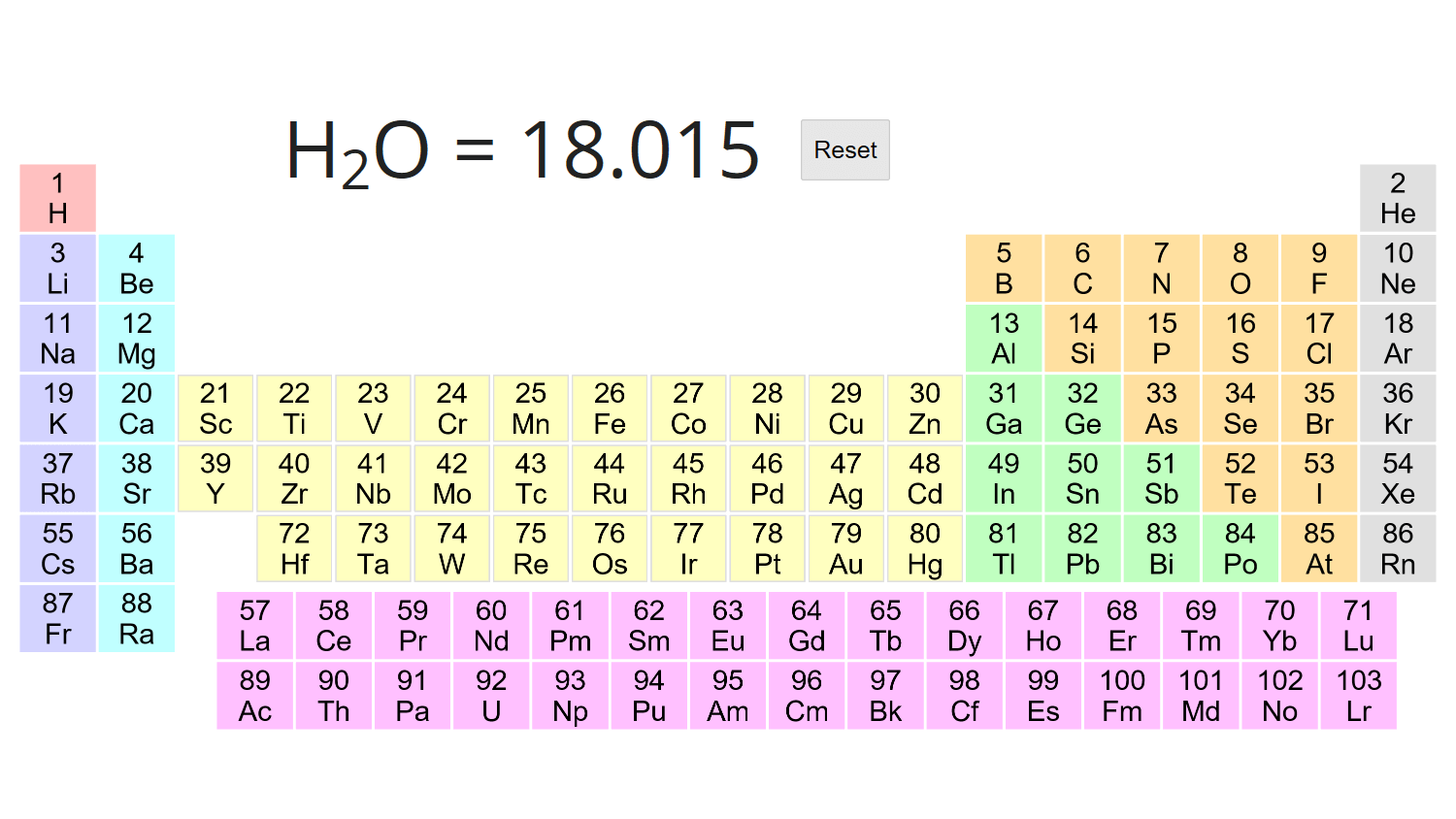
So one mole of water 6022 x 10 23 molecules has a mass of 1802 g.
. Calculating the molar enthalpy of neutralisation from experimental results is a 3 step process. How to calculate mass. These tables list values of molar ionization energies measured in kJmol 1This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions.
The molar mass is simply the mass of one mole of substance ie. For the following reaction scheme percentage yields are given along the arrow. Since the molar mass is inversely proportional to the colligative properties its value tends to be lower than expected.
IOS app is also available. Definitions of molecular mass molecular weight molar mass and molar weight. 19 K Potassium 390983.
57 to 58 C 135 to 136 F. If we have a chemical compound like NaCl the molar mass will be equal to the molar mass of one atom of sodium plus the molar mass of one atom of chlorine. 196 to 197 C 385 to 387 F.
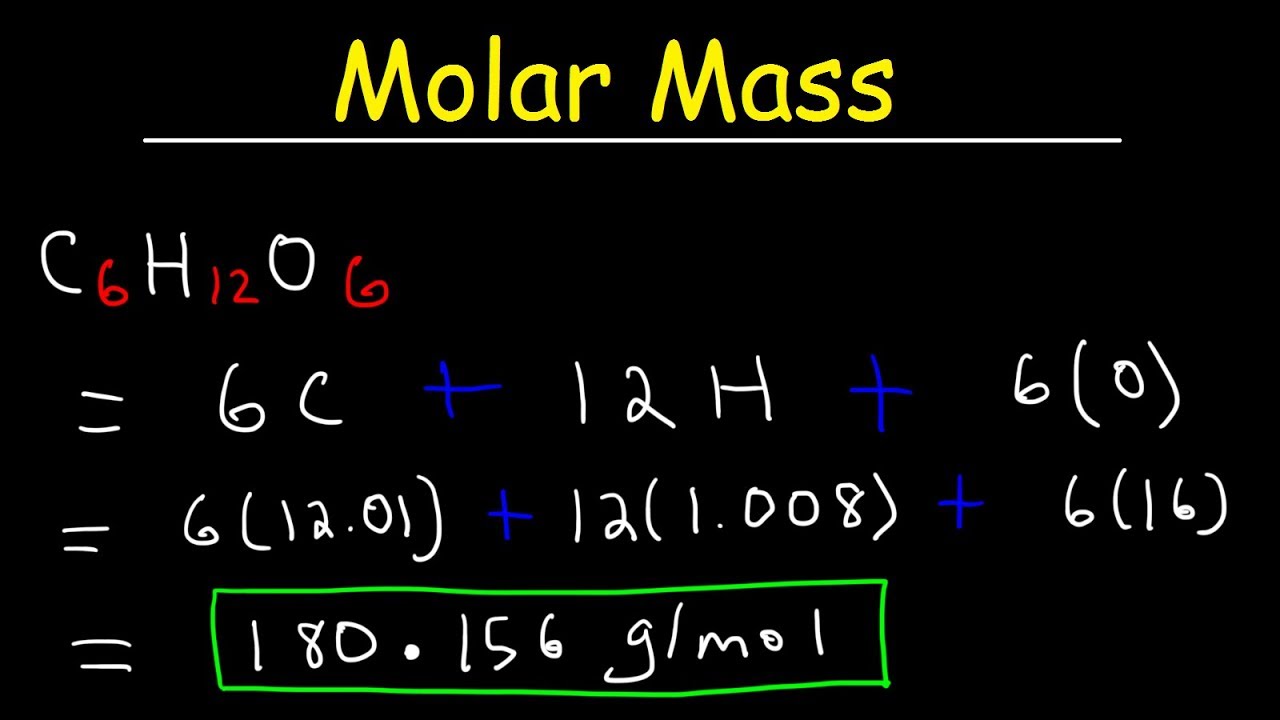
1 u is equal to 112 the mass of one atom of carbon-12 Molar mass molar weight is the mass of one mole of a substance and is. Molar Mass of Glucose C 6 H 12 O 6 Element. 469 to 470 K Solubility in water.
Calculate the heat evolved. Definitions of molecular mass molecular weight molar mass and molar weight. Options for hiding the symbol or name of the elements provide a handy learning aid for memorizing the periodic table.
The protons neutrons electrons calculator is developed to store the complete data of the atomic mass and subatomic masses of elements present in the periodic table. First calculate the molar mass of NaCl which is the mass of a mole of Na plus the mass of a mole of Cl or 2299 3545 5844 gmol Weigh out 5844 g NaCl. When solute particles associate with each other the total number of particles in the solution decreases.
In other words set the mass of each element equal to the percent. Therefore the formula. The first molar ionization energy applies to the neutral atoms.
The relation between molecular formula mass and molar mass. 18 Ar Argon 39948. 1 atom x 23 gramsmole Na 1 atom x.
Chlorine Cl 3545 u. 1 atom x 23 gramsmole Na 1 atom x. Molar mass of NaCl atomic mass of Na 2299 amu the atomic mass of Cl 3545 amu 2299 3545 5844 amu One mol of NaCl 602 x10 23 formulas has a mass of 5844 g.
If we write this as a calculation it looks like this. The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit. Finding molar mass starts with units of grams per mole gmol.
Mass g Concentration molL Volume L Formula Weight gmol. Calculate the enthalpy change for the reaction. Use the molar mass you get by adding up the atomic weight of the elements from the periodic table to convert the mass of each element into moles.
330 to 331 K Boiling point. 20 Ca Calcium 40078. If we have a chemical compound like NaCl the molar mass will be equal to the molar mass of one atom of sodium plus the molar mass of one atom of chlorine.
Molar mass in g mol 1 of H C and O as 1 12 and 16 respectivelyThe value of y is. 16338 gmol 1 Appearance Colorless to white crystalline solid Odor. One molecule of water H 2 O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a.
In other words it is incorrect to a 1 liter of water to a mass of sample to prepare a molar solution. The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number. For example the Vant Hoff factor of CaCl 2 is ideally 3 since it dissociates into one Ca 2 ion and two Cl.
Would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams. Divide each mole value by the small number of moles you obtained from your calculation. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
If we write this as a calculation it looks like this. The atomic weight of sodium is 2299 gmol and chlorine is 3545 gmol. Mass molar concentration volume and formula weight are related to each other as follows.
Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter its formula specify its isotope mass number after each element in square brackets. Molar Mass g mol 1. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
How does Atomic Mass Calculator work. C 2 H Cl 3 O 2. Use this periodic table for calculating molar mass for any chemical formula.
Place the NaCl in a 1-liter volumetric flask. The unit of molar mass in the SI system is kilogram per mole. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u.
A structure of glucose. The second third etc molar ionization energy applies to the further removal of an electron from a singly doubly etc charged ion. X g and y g are the mass of R and U respectively.
21 Sc Scandium 44955908. Q m C g T m total mass of reaction mixture C g specific heat capacity of solution ΔT change in temperature of solution Step 2. Atomic mass of H C and Cl are 1.
Examples of molecular weight computations. The flask is filled to the mark. Thus whether you type a chemical formula for a compound like CaO or type chemical symbol for a single element like Na it will show atom count molar mass and.
The molar mass of glucose is the sum of the relative atomic mass of all the atoms in the molecular formula.

How To Calculate The Molar Mass Of A Compound Quick Easy Youtube
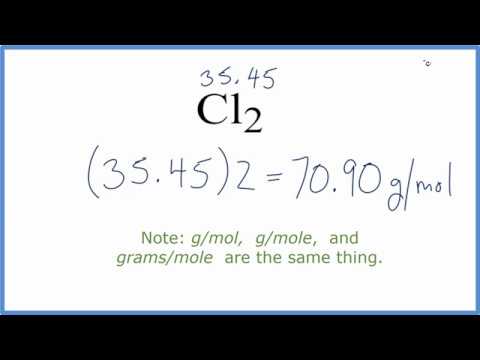
Molar Mass Molecular Weight Of Cl2 Chlorine Gas Youtube

0 Response to "Molar Mass of Cl"
Post a Comment